Get patients started with COSENTYX® (secukinumab) successfully
Suggested SC prescribing approach for appropriate patients:
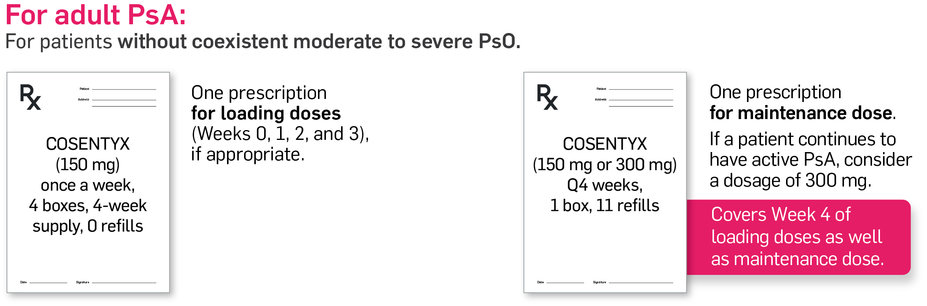
Separate loading dose and maintenance dose prescriptions1*
COSENTYX is administered subcutaneously. COSENTYX is intended for use under the guidance and supervision of a physician. Adult patients may be injected by a caregiver or self-administer COSENTYX after proper training in subcutaneous injection technique using the Sensoready® pen, UnoReady® pen, or prefilled syringe. Pediatric patients should not self-administer COSENTYX using the Sensoready pen or prefilled syringe. An adult caregiver should prepare and inject COSENTYX after receiving training on the right way to prepare and inject COSENTYX.1
Convenient SC dosing schedule
The last loading dose can be covered in the first maintenance-dose prescription.1
COSENTYX can be used alone or with methotrexate1
Recommended dosage per indication1 | ||||
|---|---|---|---|---|
Adult patients | Pediatric patients | |||
PsA | PsA with coexistent PsO | AS | nr-axSpA | JPsA/ERA |
With or without loading doses:
| With loading doses:
| With or without loading doses:
| With or without loading doses:
| With loading doses:
|
COSENTYX is intended for use under the guidance and supervision of a physician. Adult patients may be injected by a caregiver or self-administer COSENTYX after proper training in subcutaneous injection technique using the Sensoready pen, UnoReady pen, or prefilled syringe. Pediatric patients should not self-administer COSENTYX using the Sensoready pen or prefilled syringe. An adult caregiver should prepare and inject COSENTYX after receiving training on the right way to prepare and inject COSENTYX.1
*Novartis cannot guarantee that this prescribing approach is ideal for every specialty pharmacy and/or health plan. It is important to verify this information prior to filling out a prescription.
Definitions
AS, ankylosing spondylitis; ERA, enthesitis-related arthritis; JPsA, juvenile psoriatic arthritis; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis; Q4, every 4; SC, subcutaneous.
Reference
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.