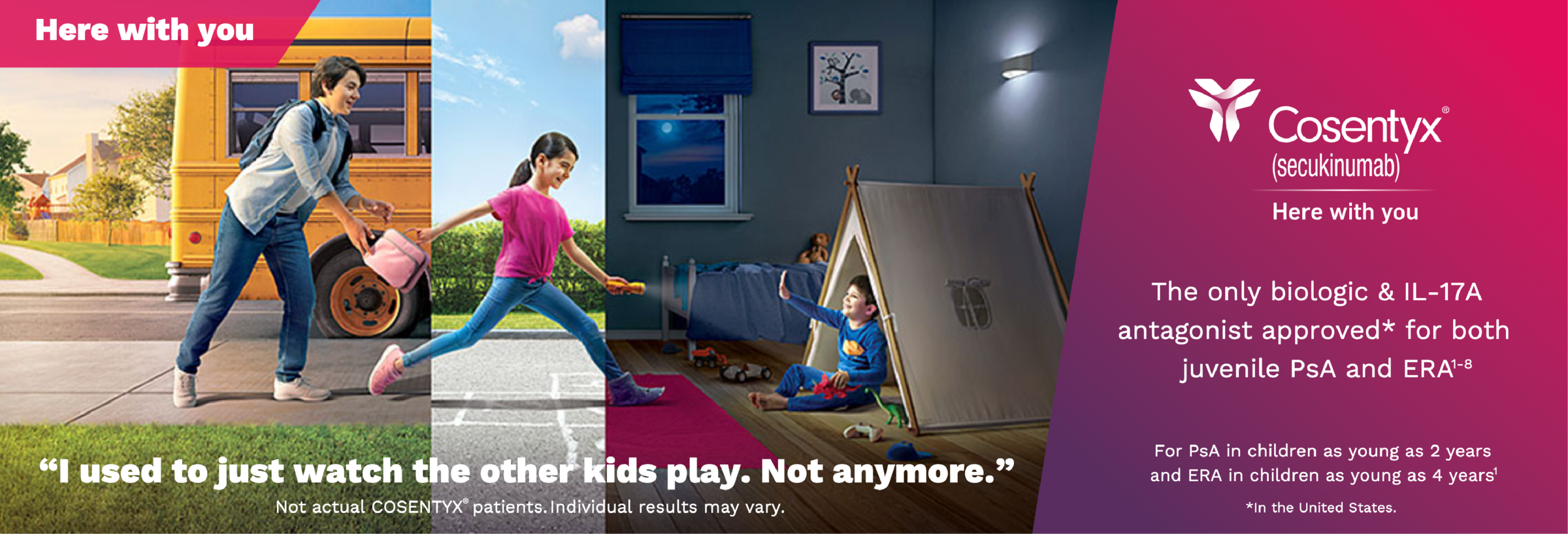Definitions
ACR, American College of Rheumatology; CHAQ, Childhood Health Assessment Questionnaire; CI, confidence interval; CRP, C-reactive protein; ERA, enthesitis-related arthritis; HR, hazard ratio; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; VAS, visual analog scale.
References
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
2. Humira. Prescribing Information. AbbVie Inc; 2021.
3. Orencia. Prescribing Information. Bristol-Myers Squibb Co; 2021.
4. Actemra. Prescribing Information. Genentech Inc; 2022.
5. Ilaris. Prescribing Information. Novartis Pharmaceuticals Corp; 2020.
6. Simponi Aria. Prescribing Information. Janssen Biotech Inc; 2021.
7. Xeljanz. Prescribing Information. Pfizer Inc; 2022.
8. Taltz. Prescribing Information. Eli Lilly & Co; 2021.
9. Data on file. CAIN457F2304 Clinical Study Report. Novartis Pharmaceuticals Corp; June 2020.
a. Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590.
b. Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report. Novartis Pharmaceuticals Corp; May 2019.
c. Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report PASI 90 and ACR Components data. Novartis Pharmaceuticals Corp; January 2020.
d. Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report mNAPSI and PASI 100 data. Novartis Pharmaceuticals Corp; October 2019.
e. Data on file. CAIN457H2315 Data Analysis Report. Novartis Pharmaceuticals Corp; April 2020.
f. Data on file. CAIN457H2315 Clinical Study Report. Novartis Pharmaceuticals Corp; November 2019.
g. Data on file. CAIN457F2310 Data Analysis Report. Novartis Pharmaceuticals Corp; June 2019.
h. Data on file. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. Novartis Pharmaceuticals Corp; February 2021.
i. Data on file. CAIN457F2342 (FUTURE 5): Interim Data Analysis Report FACIT-Fatigue data through Week 52. Novartis Pharmaceuticals Corp; April 2019.
j. Poddubnyy DA, Rudwaleit M, Listing J, Braun J, Sieper J. Comparison of a high sensitivity and standard C reactive protein measurement in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Ann Rheum Dis. 2010;69(7):1338-1341.
k. Data on file. CAIN457F2342 Clinical Study Report Interim Analysis-Week 24. Novartis Pharmaceuticals Corp; November 2017.
l. Data on file. CAIN457F2342 (FUTURE 5): 2-Year HAQ-DI biologic-naive data. Novartis Pharmaceuticals Corp; February 2021.
m. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
n. Data on file. Selected EAIRs MEASURE 2 Year 5. Novartis Pharmaceuticals Corp; January 2020.
o. Nash P, Mease PJ, McInnes IB, et al; on behalf of the FUTURE 3 study group. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20(1):47.
p. Data on file. LTD Cosentyx Prescriber and Patient Counts. Novartis Pharmaceuticals Corp; July 2021.
q. Boonen A, Sieper J, van der Heijde D, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum. 2015;44(5):556-562.
r. Data on file. CAIN457F2304 Clinical Study Report. Novartis Pharmaceuticals Corp; June 2020.
s. Data on file. CAIN457F2304 Data Analysis Report. Novartis Pharmaceuticals Corp; January 2022.
t. Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35(4):938-947.