Please see clinical trial and primary end points, key data, and study designs
COSENTYX works as early as 2 weeks with DURABLE results out to 5 years1,4
More than 2x improvement vs placebo: 61% of patients achieved ASAS20 in a mixed population at Week 16 on COSENTYX 150 mg (n=72) vs 28% on placebo (n=74) (NRI; P=0.0001) (MEASURE 2* primary end point)3,4
ASAS20 responses at Week 2 in a mixed population: 39% for COSENTYX 150 mg (n=72) and 11% for placebo (n=74)4
In MEASURE 2, ASAS20 was a prespecified exploratory end point at Week 2. No clinical or statistical conclusions can be drawn.4
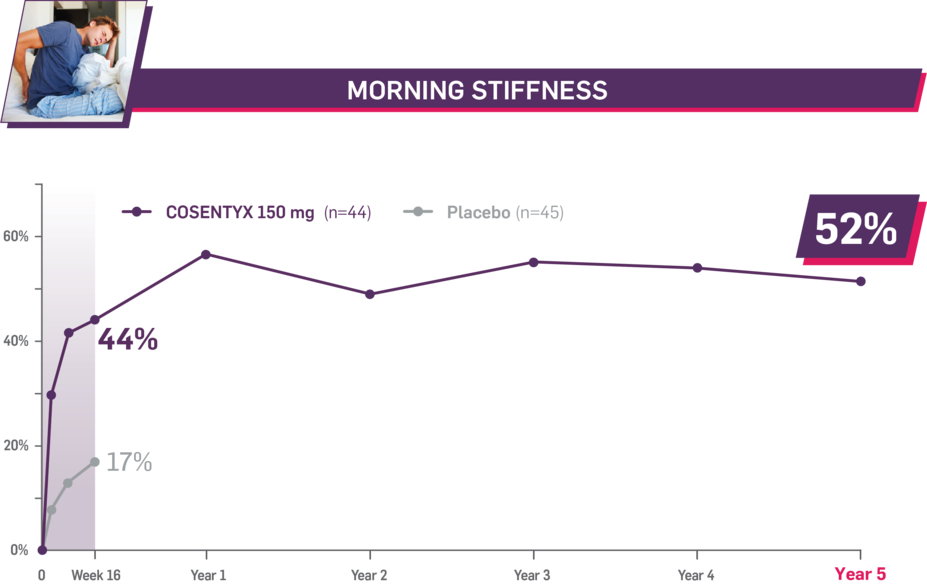
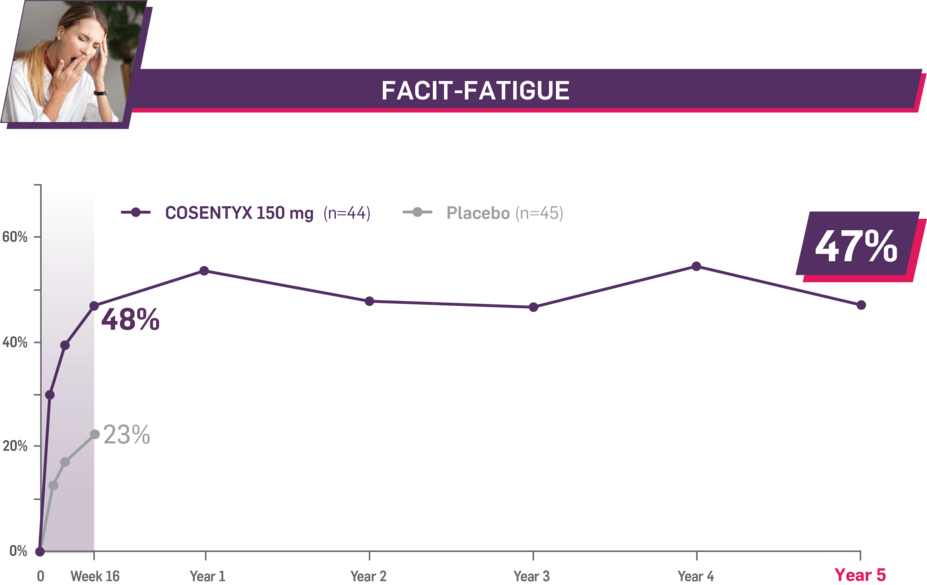
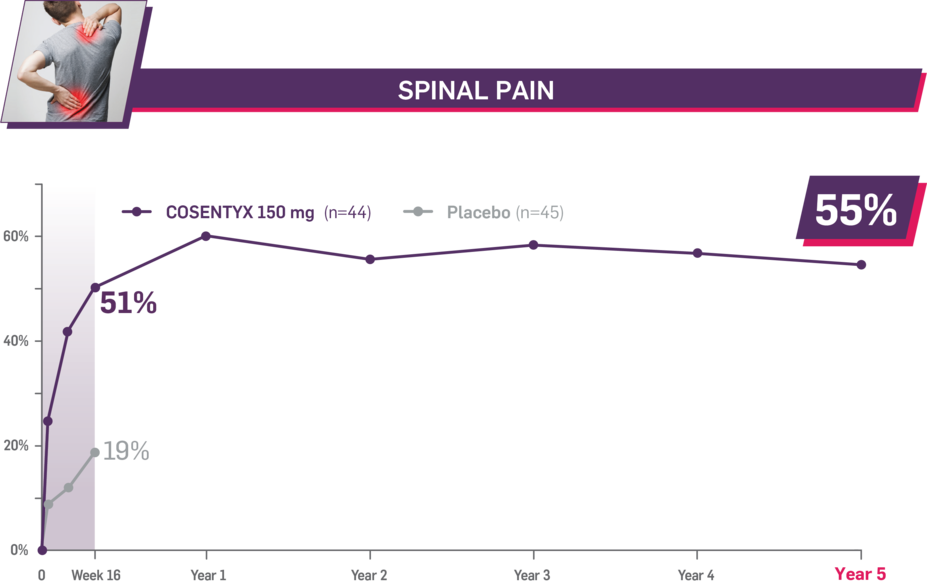
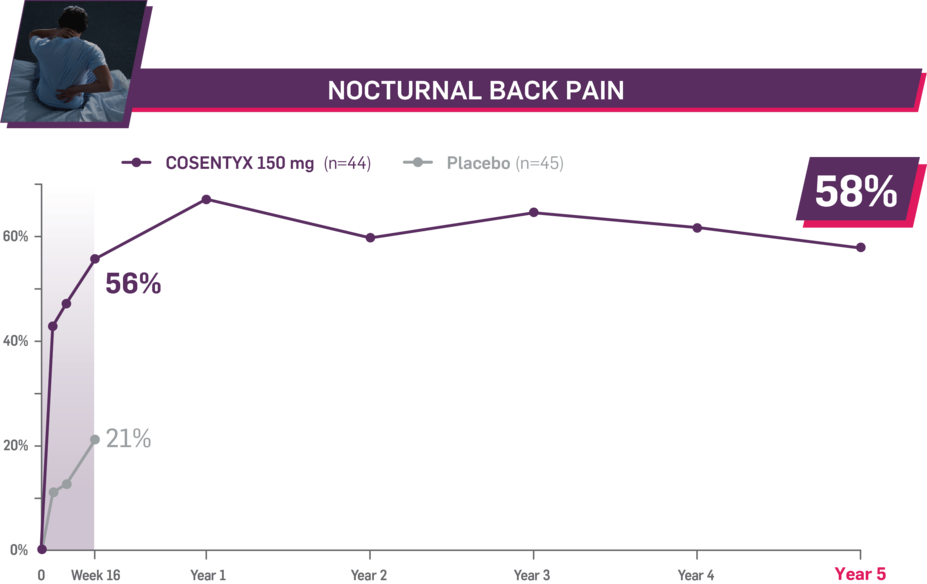
Durable improvement in mean scores in biologic-naive patients with AS (MEASURE 2; mean % for COSENTYX 150 mg)1,5
In MEASURE 2, the below measures were prespecified exploratory end points in subgroups of biologic-naive patients through Year 5. No clinical or statistical conclusions can be drawn.4
Consider escalating to 300-mg dose every 4 weeks for patients who continue to have active AS after escalating from 150 mg3
Same cost for the 150-mg and 300-mg doses‡
Mean baseline FACIT-Fatigue score at Week 16: 22.3 and 23.3 (150-mg load and placebo groups, respectively); at Year 5: 22.6 (150-mg load group). Mean change at Week 16 was 10.7 for the 150-mg group and 5.4 for the placebo group. Mean change at Year 5 was 10.6. Fatigue was measured by FACIT-Fatigue, a 13-item questionnaire that assesses self-reported fatigue and its impact on daily activities and function.1,2
Mean baseline spinal pain score at Week 16: 67.0 and 67.6 (150-mg load and placebo groups, respectively); at Year 5: 66.9 (150-mg load group). Mean change at Week 16 was −33.8 for the 150-mg group and −12.9 for the placebo group. Mean change at Year 5 was −36.8. Spinal pain was measured using a 100-mm VAS as a component of ASAS.1,2
Mean baseline nocturnal back pain score at Week 16: 66.8 and 63.9 (150-mg load and placebo groups, respectively); at Year 5: 66.8 (150-mg load group). Mean change at Week 16 was −37.4 for the 150-mg group and −13.3 for the placebo group. Mean change at Year 5 was −38.9. Nocturnal pain was measured using a 100-mm VAS as a component of ASAS.2,4,7
*Trial used SC administration.
†The effectiveness and safety of COSENTYX IV formulation are based on the pharmacokinetic exposure and extrapolation of the established effectiveness and safety of SC COSENTYX in adult patients with active PsA, AS, or nr-axSpA.3
‡Price refers to WAC. There is no increased price for higher dosage of COSENTYX.
§The updated ASAS-EULAR 2022 Guidelines are based on 2 systematic literature reviews of nonpharmacologic and nonbiological pharmacologic treatment and bDMARDs for nr-axSpA and AS. The recommendations were developed by a steering committee and task force of members from Europe and North America. Novartis is one of the many corporate supporters of the ASAS-EULAR network.6
Definitions
AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis International Society; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARDs, biologic disease-modifying antirheumatic drugs; EULAR, European Alliance of Associations for Rheumatology; FACIT, Functional Assessment of Chronic Illness Therapy; IL, interleukin; IV, intravenous; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; SC, subcutaneous; VAS, visual analog scale; WAC, wholesale acquisition cost.
References
1. Data on file. CAIN457F2310 (MEASURE 2): Data Analysis Report. Novartis Pharmaceuticals Corp; June 2019.
2. Data on file. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. Novartis Pharmaceuticals Corp; February 2021.
3. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
4. Data on file. CAIN457F2310 (MEASURE 2): Clinical Study Report. Novartis Pharmaceuticals Corp; November 2014.
5. Data on file. Conversion data tables for ankylosing spondylitis. Novartis Pharmaceuticals Corp; June 2019.
6. Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19-34.
7. Data on file. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. Novartis Pharmaceuticals Corp; January 2020.